
 Stable Isotopes in Water
Stable Isotopes in Water
Water has the chemical formula H2O. The isotopic composition of both the Hydrogen and the Oxygen in water provides useful information about the sources of the water sample, or its interaction with the environment.
For example, evaporation of water favors water molecules with light oxygen (16O) and light hydrogen (1H or just H) over those with heavy oxygen (18O) or deuterium (2H or D). Hence water vapor is enriched in the lighter isotopes 16O and H relative to the liquid water left behind. There is great variation in the stable isotope composition of precipitation with increasing latitude or altitude due to differences in local temperatures and evaporation rates. At any given location the isotopic composition of precipitation varies temporally between seasons (Fig. 1) and from storm to storm, as well as spatially within the soil profile.
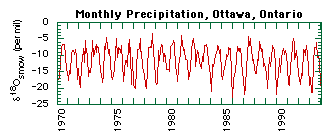
Figure 1: The oxygen isotope composition of monthly precipitation at Ottawa, Ontario between January 1970 and December 1993. Although Ottawa is the closest GNIP (Global Network for Isotopes in Precipitation) station to Keene, it is still too far away to be representative of local conditions.
To analyze the oxygen isotopes in water, the headspace over the water in a sealed, evacuated tube is filled with CO2 gas. An isotopic exchange reaction takes place between the water and the CO2, while held at a constant temperature. After equilibrium has been obtained, the CO2 is extracted for analysis on the mass spectrometer. To analyze the hydrogen isotopes in water, the water is reduced with metallic zinc in a sealed, evacuated tube at high temperatures, liberating H2 gas.
Website by ThorpeAllen.net
Last modified 2008-12-31