
 ydrogen Isotopes
ydrogen Isotopes
Hydrogen has two naturally occurring stable isotopes (1H or just H, and 2H or D for Deuterium), and one naturally occurring radioactive isotope (3H, or T for Tritium). For studies of stable isotope biogeochemistry we measure the ratio of D to H in samples of interest, expressed relative to the ratio in a standard of known isotopic composition, usually "Vienna Standard Mean Ocean Water" as prepared by the International Atomic Energy Agency in Vienna (V-SMOW; D/H = 0.00015595 (1)(2)).
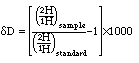
The dD values of terrestrial hydrogen samples (as in water) typically range from -450 to +50 per mil relative to V-SMOW (1).
The KSC Center for Environmental BioGeoChemistry is not equipped to measure Tritium concentrations.
| Isotope | Atomic mass (ma/u) |
Natural abundance (atom %) |
|---|---|---|
| 1H | 1.007825035 | 99.9885 |
| 2H or
D (Deuterium) |
2.014101779 | 0.0115 |
| 3H or
T (Tritium) |
3.01604927 | half-life of 12.32 years
decays by b- to 3He |
See the ![]() page on the
Isotopes of
Hydrogen.
page on the
Isotopes of
Hydrogen.
and the ![]() centered on H.
centered on H.
(1)From Table 9-1, p. 154, of David P. Mattey, 1997, Gas source mass spectrometry: isotopic composition of lighter elements, pages 154-170 in Robin Gill (editor), Modern Analytical Geochemistry, Longman (UK).
(2)The uncertainty in this value is much larger than the precision with which we can measure relative ratios, which is one reason for the use the "delta" notation; page 32 in Robert E. Criss, 1999, Principles of Stable Isotope Distribution, Oxford University Press.
Website by ThorpeAllen.net
Last modified 2008-12-31